How Revolutionary 'Bioprinting' Can Produce Living Organs From Scratch
So we all know about that newfangled, cutting-edge 3D printing, right? Okay, maybe it's not newfangled anymore. 3D printers are so commercially common that PC Magazine has a "best 3D printers for 2023 list," with the cheapest as low as $300 — and that's a kid's plaything version. Format lists lots of the tiny kitsch you can make with small-scale 3D printers at home: keyholders, tiny planters, phone stands, combs, bookmarks, popsicle molds, and so forth. Basically, a whole bunch of dollar-store junk to clutter up your once-pristine living space. Big industrial printers like those on 3D Systems are a whole different story, though, cooking up complex plastic objects, metal objects, jewelry, whole dentures, and more.
But how about some truly wild, revolutionary, WTF technology like printing new body parts or organs? Yes, for real. Forget artificial limbs and cyborgs and consciousness uploads into robot bodies. You need a new aorta? Boom: We'll whip one up. Oh, you lost a finger? Stick around and we'll grow you another. You've got a faulty liver? Hang on, let's give you a scan and print you a new one — there's the waiting room. That sounds like some kind of bonkers science fiction, but it's not. At least it won't be science fiction given enough time and investment and resources. Bioprinting, as it's called, allows us to print new, living tissue using constituent elements from patients and the environment around us: algae, shrimp, skin, bones, blood, and more.
Evolution at breakneck speed
Of course bioprinting is a new thing, but it's probably less new than readers realize. Before any technology makes its way to the public in any kind of commercially viable way, it circulates internally amongst researchers, engineers, investors, tech startups, material scientists, etc. This is why journals like Biomaterials Science have articles about bioprinting dating back to 2018. These things occur and evolve rapidly with the public being none the wiser.
We've already got whole companies devoted to producing the hardware, software, bioink and hydrogels (more on those later), and all other implements involved in the entire bioprinting process, like Inventia, TissueLabs, CELLINK, Merck, Nano Dimension, and more. Some sell bioprinting products to other companies, some make the physical printers, others investigate more efficient materials used when printing, etc. CELLINK alone has multiple trademarked models of bioprinters like Bio X, Bio X6, and INKREDIBLE+. To those working in this field, this is all old news.
Quoting Nano Dimension, Engineering.com succinctly sums up the rollover from 3D printing to bioprinting thusly: "Think about it. What's a tissue? It's a set of cells made up mostly different cell types, placed together in an orderly manner. They might have some other materials combining them or holding them in one way or another. These materials could be proteins or other structural and connective elements. One tissue might be soft, while another could be firm. Together, different tissues form an organ."
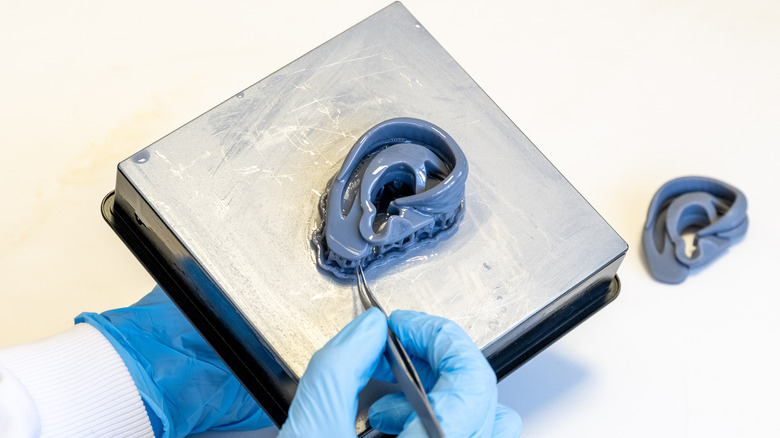
Key terms: bioink and hydrogel
So how does the whole bioprinting thing work, exactly? Ultimately, those folks at Nano Engineering were right: It's not too different from 3D printing. In fact, 3D printing isn't too different from printing ink on paper, except that a 3D-printed object becomes "3D" because it keeps getting layers added to it, up, up, until it makes a complete object. That kind of 3D printing is called "extrusion-based," as CELLINK explains, and is generally regarded as the most versatile way to either 3D print or bioprint. There are other types, too, that for brevity's sake the reader can explore independently: inkjet-based, laser-based, stereolithography, etc. Each has particular caveats, drawbacks, advantages, and functions.
As for materials, bioink stands at the forefront. If you think of a piping bag for squeezing frosting onto a cake, then you'll understand bioink. But rather than sugary pink frosting, bioink is made from fundamental cellular building blocks like collagen (from skin and ligaments), chitosan (carbohydrates found in shellfish), agarose (from seaweed), alginate (from brown algae), fibrin (produced when blood clots), gelatin (that stuff that makes gummy bears gummy), etc. In other words, bioink is an "extracellular matrix," the stuff around and between cells that lets them work and keeps them connected. As Science News Explores explains, bioink comes suspended in hydrogels — gel-like substances that convey and house the ink. As Inventia outlines, you shoot the bioink on a plate in a programmed pattern, grab the popcorn, and wait.
From biopsy to body
Merck gets into some details about bioprinting, including a flow diagram, tables of bioinks and their advantages and disadvantages, and tables of the same for printing methods. Once we move past the generalities of "print a material on a surface," things get complicated — fast. But we can take one example — the bioink Gelatin Methacryloyl (GelMA) derived from gelatin — to truly appreciate the brilliance of all those involved in the entire bioprinting process. GelMA is used to build cardiomyocytes (heart cells that make the heart beat), the endothelium (inner lining of blood vessels), the epidermis (outer skin layer), cartilage, bones, and "injectable tissue constructs," i.e., matrices of cells that can be shot into deeper tissue layers, where they bloom into shape.
And if you're wondering how a random bioprinted organ on a plate could be compatible with any given person, bioprinting begins with a patient. Dr. Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine, told CNN that doctors start by taking a biopsy of a patient's own cells "less than half the size of a postage stamp." From there, researchers separate cells of different types and keep them in incubator-like containers fed with nutrients. Doctors then mix these cells with bioink, which contains all the fundamental proteins, collagen, acids, etc., that cells need to function. It might be obvious, but bioink needs to be non-toxic, biodegradable, and biocompatible to avoid producing a response from the immune system. As for how the cells grow: They do that on their own.
A brave, bioprinted future?
It should go without saying, but bioprinting could overhaul regenerative medicine head to toe. A 2016 study from the National Science Review mentions bioprinting's most obvious application: bypassing organ donors. The Global Observatory on Donation and Transplantation (GODT) says that there were about 144,000 organ transplants worldwide in 2021, or 16 transplants per hour. But wait lists continue to grow and there aren't enough donors — the Health Resources and Services Administration (HRSA) says that 17 people die every day waiting for an organ. Meanwhile, 3Dnatives says that researchers in Brazil grew a small liver in 90 days using a sample of a patient's blood. Bioprinting could ensure that no one dies while waiting for an organ, and also obliterate the illegal organ trade.
But as always, there are financial and ethical concerns. CNN says that bioprinted organs would be substantially cheaper than transplants. Yet given that the average cost for a kidney transplant in the U.S. in 2020 was a mindboggling $442,500, "cheaper" might not be saying much. Would bioprinting help the world fracture even faster into upper and lower castes of youthful and healthy vs. aged and indigent? Also, bioink materials have to come from somewhere. Are we going to strip-mine Earth for even more resources, harvesting algae and shellfish to extinction, or descend to a horrid recycle-the-poor-to-sustain-the-wealthy dystopia? Thankfully, there's still time to help bioprinting meet its full, benign potential, provided the public knows and cares.