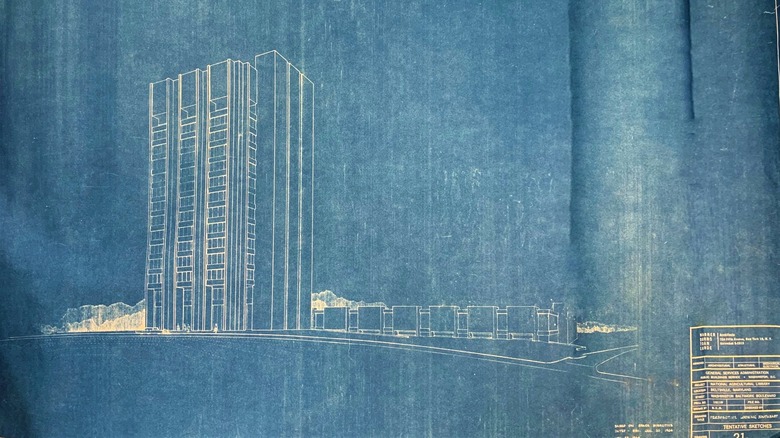
Are Blueprints Actually Blue?
The history of architecture dates way back to the Neolithic period, the final stage of the Stone Age when significant developments occurred. It was around that point in time when people started constructing their own dwellings instead of just taking shelter in existing caves (via World History). Back then, constructing structures was done for survival. As the years passed, however, building construction evolved to include scientific and artistic aspects — what we now know as architecture.
The earliest known samples of structural drawings date back to 2200 B.C., and they were used as a means for builders to determine the requirements for building structures. Fast forward to the 13th century and architectural drawings became a more accurate representation of what was to be built, as reported by Arch Daily. The earliest drawings were monochromatic sketches that were mostly created to provide workers with information about the building. And then, blueprints were invented.
The history of blueprints
In 1842, astronomer, photographer, and chemist John Herschel created the process of blueprinting. The process starts with drawing the design on semi-transparent paper placed over cloth or a sheet of paper that is treated with photosensitive chemicals. Light is then shone on the paper for several minutes, and the sheet turns blue, while the lines on the drawing remain white. The process creates a negative drawing — known as the blueprint — of the original sketch, as explained by the Polymer Science Learning Center.
The question is, why does the paper turn blue? The chemicals used in the process include ferric ammonium citrate and potassium ferricyanide and when combined, the chemical reaction results in a blue color — a compound called blue ferric ferrocyanide, according to How Stuff Works. Before blueprints, drawings were hand-traced, which was a tedious and time-consuming process. With the faster process made available, blueprinting became the primary process of image reproduction in the 19th and 20th centuries.
Prussian blue
Blue ferric ferrocyanide — the photosensitive chemical used in blueprinting — is also called Prussian blue. It was accidentally discovered in the early 18th century by a dye maker named Diesbach while he was in the process of creating red dye. Diesbach added potash to the mixture and ended up with a deep, blue color, per How Stuff Works. It turned out that the ingredients he used caused a chemical reaction that resulted in vibrant blue.
Prussian blue is called such, as it was the primary compound that was used to dye the uniforms of members of the Prussian army. The pigment is used today to color textiles, paints, and inks, but the compound is also used in sodium-ion battery electrodes, as reported by the American Chemical Society. Prussian Blue was also one of the original colors released by Crayola (then known as Binney & Smith Company) in the early 20th century, but the name was changed to Midnight Blue in 1958.