A History Of The Search For Water On Other Planets
Every couple of years or so, the scientific community tends to excitedly blurt, "We found water!" on some other planet or moon. In January 2022, astronomical hearts were aflutter at the possibility of water existing in the atmosphere of gas giant TOI-674 b, an exoplanet (planet in another solar system) about 150 light-years from Earth (via NBC). In 2019, the exciting hydropathic news came from exoplanet K2-18 b, about 110 light-years away in the constellation Leo. K2-18 b might even have a surface temperature suitable for flowing, liquid water (via Scientific American). And let's not forget the endless quest for water on our neighboring planet Mars (via Harvard), moons of Jupiter and Saturn like Titan, Ganymede, Enceladus, and Europa (via the National Ocean Service), and even our own rocky moon (via NASA).
All in all, it looks like the search for water is an astronomical near-obsession of ours, on par with the search for alien life and habitable planets. And indeed, those searches often go hand-in-hand. It makes sense that we would, from our beautiful blue ball of dwindling freshwater and toxifying, superheated oceans, seek out planets as full of life-giving water as our own. If we need to take off into space to escape Earth or colonize in general, we've got to have water. And if extraterrestrial life at all resembles us, they might need it, too. But before the galactic water wars commence, let's have a look at the short, but involved, history of the quest.
The universal solvent of life
By now, we've all heard plenty of anecdotes about human dependency on water: we're mostly made of the stuff (60-70%, per the BBC), we'll die within days without it (three to four days is the usual limit), you need to drink 8 glasses of it a day (it's actually 3.7 liters/day for men, 2.7 liters/day for women, per MayoClinic), and so on. Besides such folk facts, it's not too difficult to simply observe the natural world: dew on morning grass, clouds in the sky, rivers flowing down from mountains to seas, etc. Within Earth's circulatory system, water is one of the chief substances that binds life together.
But why, exactly, is water so integral to life? It is, as Harvard University states, the "universal solvent." Its molecular structure — one atom of oxygen and two atoms of hydrogen — makes it the ideal medium to bind with and pass through other molecules. What it doesn't bind with — it houses. What it can't house it separates. The presence of water, inside and outside of cells, supports the very structure of cell walls, and the apparatuses that cells use to process amino acids, proteins, enzymes, and so forth. Water also conducts electricity and can, more or less, infinitely bind with itself.
This is why astrobiologists say that we should "follow the water" when looking for extraterrestrial life. If alien life operates like us, then places with water could not only sustain alien life but humanity, as well.
Backyard water supplies
The search for water on other planets is a relatively new phenomenon and has largely been confined to our own solar system. Even though scientists such as Galileo first spotted Jupiter's moon Ganymede in 1610 (via NASA), he probably didn't sit around and think, "Hm, I wonder if I can take a swim up there." Since then, the search for water has evolved organically, and until the past 20 years or so, most discoveries have been incidental.
Our closest cosmic buddy — the moon — was late to the water discovery party, even though astronauts walked on it back during the Apollo mission in 1969. As they rightfully pointed out, the moon is a craggy, dry ball without much going on. And yet, as NASA continues, they dispatched their LCROSS satellite (Lunar Crater Observation and Sensing Satellite) towards the moon's poles in 2009. Lo and behold: there's permanently frozen ice on the moon's poles.
Since then, the search for lunar water has even revealed ice in direct sunlight on the light side of the moon. In 2020, as NASA explains, their SOFIA observatory did a close scan of the moon's Clavius Crater, one of the largest craters visible from Earth. They discovered water to the tune of 100 to 412 parts per million (about a 12-ounce water bottle). Granted, this isn't enough to sustain a moon colony, but it illustrates that water can exist in the most unlikely places.
The red planet's underground stash
One step past the moon takes us to Mars. And no, forget about water anywhere closer to the sun. Venus is such a scorched and sulphuric hell that, "even the most drought-tolerant of Earth's microbes wouldn't be able to survive," as Scientific American describes. Mercury is close enough to the sun to get blasted with heat to the tune of 427 degrees Celsius (800 degrees Fahrenheit) on its light side, via Space. So no, no water there.
In comparison, Mars — however dry, dusty and red it is — looks downright hospitable. It may not be home to little green men bobbing up and down while waving "Hello Earthling" signs to NASA's Curiosity or Perseverance land rovers, but we've discovered that it does, indeed, house water.
As Vice explains, as recently as 2021 the European Space Agency (ESA) discovered a massive ice reservoir lodged underground in Mar's largest set of canyons, Valles Marineris (Latin for "Mariners Valley"), also known as "The Grand Canyon of Mars." The ESA's FREND instrument (Fine Resolution Epithermal Neutron Detector) made the discovery from orbit, itself attached to the ESA's Trace Gas Orbiter satellite.
FREND spotted an unusual patch of hydrogen emissions in Valles Marineris that indicated water in a reservoir that's, "large, not too deep below ground, and could be easily exploitable for future explorers." It makes sense that the bottom of a deep, trench-like canyon would afford easier access to an underground water supply.
In the orbits of giants
The largest supplies of water in our solar system dwell in orbit around our solar system's two biggest planets, Jupiter and Saturn. Two of their moons, each, are quite literally flush with water.
We've known about water on Europa, one of Jupiter's moons, since the Voyager spacecraft passed by in 1979 (via NASA). Europa doesn't just have a little water, but an entire shell of ice covering the planet. It's one-fourth the dimensions of Earth but could contain up to twice the water on our planet. Ganymede, on the other hand — the largest moon in our solar system — has a powerful magnetic field likely caused by subterranean, liquid saltwater oceans (via NASA). In 2014 researchers concluded that the combination of water and salt could even allow for the development of life, according to NASA.
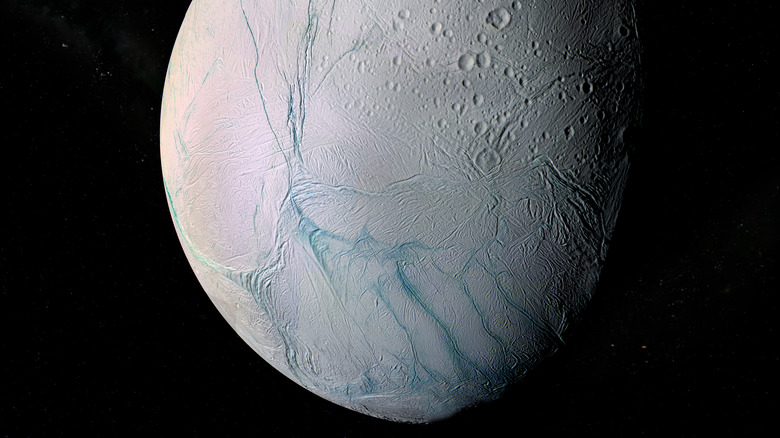
In 1980, scientists noticed that Saturn's moon Enceladus happened to be excessively, blindingly white (via NASA). The whole surface, it turns out, is one, smooth ice sheet. Because of thermal pressure, it even jettisons water into space. Saturn's mammoth moon Titan, larger than the planet Mercury, goes one step further by having actual lakes, rivers, oceans, clouds, rain, and more (via NASA). Granted, much of this weather cycle is composed of methane and ethane, but Titan also has oceans of H2O, an entire crust of ice, and more water trapped under the surface. It took until 2005 to peer beyond the moon's hazy atmosphere.
Light years beyond Earth
Beyond the icy moons of Jupiter and Saturn, we've got the icy planets of Uranus and Neptune. Both of them, as Astronomy points out, are referred to as "ice giants" because they're composed of solid ice cores encased in mantles of "compressed, slushy water and ammonia." And beyond Uranus and Neptune, as NASA explains, our entire solar system is ringed in the Kuiper Belt, a disc of billions of icy, comet-like fragments left over from our solar system's creation (they didn't get gravitationally squashed into planets or moons).
With all of this water hanging around, and much of it recently discovered — but discovered without too, too much difficulty — the question begs: are all solar systems so replete with water? Truthfully, it would be a mistake to believe that the entire cosmos is riddled with water simply because of the conditions of our immediate celestial neighborhood. That's like living on a beach, never having left the beach, and therefore assuming that the whole Earth is composed of beaches. As Quanta Magazine points out, H2O of the solid-liquid-gaseous type might be the rare exception to a universe-wide, hot, black, very undrinkable "superionic ice."
Recent, light-years-away discoveries of water on exoplanets TOI-674 b and K2-18 b bode well enough for the galactic search. That being said, no water outside of the Earth is as easily obtainable as what flows from your tap. Should we keep our eyes to the stars? Sure. But we should also best preserve what we have.